

Modafinil is a eugeroic (wakefulness-promoting agent) that was developed in the 1970s as a treatment for narcolepsy, and has since shown great potential for many medical – and non-medical – uses. It is now registered for use in the treatment not just of narcolepsy, but of shift work sleep disorder and excessive daytime sleepiness associated with obstructive sleep apnoea. And its excellent safety profile and low risk of abuse have led to widespread off-label use as well.
As the word has spread and modafinil has made its way into the media, more and more healthy people have been taking advantage of modafinil’s perceived beneficial effects on wakefulness, focus, and creativity. But modafinil remains a scheduled drug, and using it without a prescription is not legal. Moreover, controlled studies in healthy people remain scarce, so while the safety of the drug is considered to be high, we shouldn’t throw caution to the wind just yet.
>> Looking to Buy Modafinil? Get it here for less than $1 per pill!
Here we take an in-depth look at the history of the drug, its on- and off-label uses, its nootropic effects, and what is known about how it works, how it is absorbed and eliminated, and side effects and safety concerns. We conclude with a brief treatment of general considerations before taking modafinil.
History of modafinil

Modafinil was discovered in 1976 by French neurophysiologist Michel Jouvet in collaboration with Lafon Laboratories. Jouvet called the drug ‘a great French discovery’, adding that he had used it himself and experienced greater productivity[1]Cahill, M. (2005). The Ethical Consequences of Modafinil Use. Penn Bioethics Journal Vol. I, Issue 1.. It was one of several benzhydryl sulfinyl compounds, including adrafinil, being invented and trialled at the time. After adrafinil was found to cause hyperactivity in animal studies, it was trialled as an experimental treatment for narcolepsy in France in 1986. However, it wasn’t long before adrafinil was discontinued in favour of its active metabolite, modafinil, which is faster-acting and more well-absorbed than adrafinil.
Modafinil has been prescribed in France since 1994, in the US since 1998, in the UK since 2002, and in Australia since 2005. It can now be obtained with a prescription in most first-world countries. In the United States and Australia, modafinil is a Schedule IV drug, meaning it is restricted in availability and usage due to concerns about the potential for abuse and addiction even though research has not supported such claims.
More recently, a new ‘version’ of modafinil has hit the shelves – armodafinil. Modafinil consists of two enantiomers – mirror-image versions of the same molecule. Armodafinil is a purified version of modafinil that contains only one of these enantiomers, known as R-modafinil. Now known as armodafinil, this drug has the same uses and effects as modafinil, but reaches its peak concentration later and has a longer half-life (15 hours versus 12 hours), so maintains higher blood levels and therefore exerts stronger effects throughout the day. However, in practice, the difference in effects is often not significant.
Both modafinil and armodafinil are produced by Cephalon and marketed as Provigil and Nuvigil respectively. However, modafinil is now being produced in multiple generic forms, whereas armodafinil remains exclusive to Cephalon.
Registered medical uses of modafinil
Modafinil is registered for the treatment of narcolepsy and excessive daytime sleepiness associated with shift work sleep disorder and obstructive sleep apnoea/hypopnoea. Daytime sleepiness can stem from a variety of causes, and the utility of modafinil in treating these conditions depends on the nature of the specific disorder.
Here we overview the existing research on the use of modafinil in a range of conditions causing sleepiness and fatigue, as well as some novel uses in other disorders. Modafinil was well tolerated with a low incidence of side effects and no signs of dependence in all the studies discussed below. Therefore, these aspects will not be discussed for each disorder.
Narcolepsy

Narcolepsy is a condition characterised by daytime sleepiness and an extreme tendency to fall asleep during the day. It may also involve cataplexy (sudden collapse) and sleep disturbances, including sleep paralysis and hallucinations. While its causes have not been fully elucidated, part of the mechanism appears to be the absence of certain neurons in the brain that release the neurotransmitter orexin, which is involved in the regulation of wakefulness[2]Nishino S., Kanbayashi T. (2005). Symptomatic narcolepsy, cataplexy and hypersomnia, and their implications in the hypothalamic hypocretin/orexin system. Sleep Med Rev. 9, 269-310.. With multiple studies demonstrating its efficacy, modafinil has become the first line of treatment for narcolepsy[3]Billiard, M. (2008). Narcolepsy: current treatment options and future approaches. Neuropsychiatr Dis Treat. 4, 557–566..
Two double-blind, placebo-controlled, crossover studies (N = 50[4]Billiard, M., Besset, A., Montplaisir, J., Laffont, F., Goldenberg, F., Weill, JS., Lubin, S. (1994). Modafinil: a double-blind multicentric study. Sleep. 17, S107-12., N = 75),[5]Broughton, RJ., Fleming, JA., George, CF., Hill, JD., Kryger, MH., Moldofsky, H., Montplaisir, JY., Morehouse, RL., Moscovitch, A., Murphy, WF. (1997). Randomized, double-blind, placebo-controlled crossover trial of modafinil in the treatment of excessive daytime sleepiness in narcolepsy. Neurology. 49, 444-451. examined the effects of 200, 300, and 400 mg/day doses of modafinil on narcoleptic patients. In both studies, participants had reduced daytime sleepiness, with no compromise to nighttime sleepiness.
The US Modafinil in Narcolepsy Multicenter Study Group later conducted two larger, randomised, double-blind controlled trials (N = 283[6]Fry. (2004). Randomized trial of modafinil for the treatment of pathological somnolence in narcolepsy. US Modafinil in Narcolepsy Multicenter Study Group. and N = 271)[7]Randomized trial of modafinil as a treatment for the excessive daytime somnolence of narcolepsy: US Modafinil in Narcolepsy Multicenter Study Group. (2000). Neurology. 4, 1166-1175. that both showed efficacy as early as week 3 in multiple measures of daytime sleepiness. When these trials were continued, albeit without blinding, for up to 136 weeks, modafinil showed continued effectiveness, and few patients discontinued the medication[8]Ballon, JS., Feifel, D. (2006). A systematic review of modafinil: Potential clinical uses and mechanisms of action. J Clin Psychiatry. 67, 554-566.. In another long-term open-label study (N = 140) that monitored narcoleptic patients receiving modafinil for up to 114 months, 64% of patients rated the effect of modafinil on daytime sleepiness as good or excellent[9]Besset, A., Chetrit, M., Carlander, B., Billiard, M. (1996). Use of modafinil in the treatment of narcolepsy: a long term follow-up study. Neurophysiol Clin. 26, 60-66..
Shift work sleep disorder

Shift-work sleep disorder occurs when our circadian rhythm is disturbed by a work routine that does not follow the usual human sleep-wake cycle. For those who work night shifts, either continually or intermittently, it can be difficult for the body to adjust to this abnormal pattern of sleep and wakefulness. As a result, shift workers often experience excessive sleepiness when they need to stay awake at night, and insomnia when they try to sleep during the day. Resumption of a normal sleep-wake cycle can alleviate these problems, but when your job requires you to work these hours, there’s no choice. Overall, the research suggests that while modafinil may have some utility in alleviating the symptoms of SWSD, it is far from a perfect solution.
In a 2005 multi-centre, double-blind, placebo-controlled study (N = 209), participants with SWSD took 200 mg/day of modafinil over 12 weeks[10]Czeisler et al. (2005). Modafinil for Excessive Sleepiness Associated with Shift-Work Sleep Disorder. N Engl J Med, 353, 476-486.. The researchers documented improved alertness throughout the night, longer sleep latency (time taken to fall asleep), and almost half as many accidents or near-misses on the drive home compared with the placebo group. However, participants were just as likely to fall asleep or have accidents at work and consumed just as much caffeine during and before the shift as those receiving a placebo.
A 2003 double-blind, placebo-controlled study (N = 278) that assessed quality-of-life measures in people with SWSD found that modafinil was associated with better sleep quality and self-assessed mental health[11]Erman, M., Rosenberg, R. (2007). Modafinil for Excessive Sleepiness Associated With Chronic Shift Work Sleep Disorder: Effects on Patient Functioning and Health-Related Quality of Life. Prim Care Companion J Clin Psychiatry. 9, 188–194.. However, an open-label (non-blinded) study (N = 118) conducted over one year demonstrated no difference in sleep quality outcomes between modafinil and placebo treatment groups[12]Ballon, JS., Feifel, D. (2006). A systematic review of modafinil: Potential clinical uses and mechanisms of action. J Clin Psychiatry. 67, 554-566..
Sleep deprivation among soldiers represents a specific form of SWSD with exceptionally high stakes: in combat situations, lapses in concentration could prove fatal. Traditionally, armed forces have used amphetamines and other stimulants where necessary to maintain alertness in soldiers who are required to remain awake for lengthy periods. But in France, where modafinil was discovered, the military started using modafinil to boost mood and cognition in soldiers as early as 1991. The UK has been investigating its utility in military contexts since the late 1990s,[13]Matthews, R. (2008). Sleepless in battle. Science Focus. and the US approved its use among Air Force pilots in 2003[14]Caldwell, JA., Caldwell, JL., Smith, JK., Brown, DL. (2004). Modafinil’s effects on simulator performance and mood in pilots during 37 h without sleep. Aviat Space Environ Med. 75, 777-784.. Canada, China, the Netherlands, South Korea, Taiwan, Singapore, and India are now all experimenting with its use in military applications[15]Saletan, W. (2013). The War on Sleep. Slate. Modafinil has also been used by astronauts aboard shuttles and the ISS.[16]Barger et al. (2014). Prevalence of sleep deficiency and use of hypnotic drugs in astronauts before, during, and after spaceflight: an observational study. The Lancet Neurology Volume 13, No. 9, p904–912..
Obstructive sleep apnoea/hypopnoea

Obstructive sleep apnoea/hypopnoea (OSAH) is characterised by partial or complete obstruction of the airways during sleep resulting from the collapse of the upper airways. Affected people typically snore and/or stop breathing intermittently throughout the night, sometimes causing them to wake up. This disruption of normal sleep can lead to excessive sleepiness during the day. It may also be associated with an increased risk of cardiovascular disease[17]Golbin, J., Somers, V., Caples, S. (2007). Obstructive Sleep Apnea, Cardiovascular Disease, and Pulmonary Hypertension. AnnalsATS Issues Vol. 5, No. 2.. While it cannot directly alleviate the airway obstruction that characterises OSAH, modafinil may have a role in ameliorating the daytime sleepiness that goes along with it.
A 2005 double-blind, placebo-controlled study (N = 309)[18]Black, JE., Hirshkowitz, M. (2005). Modafinil for treatment of residual excessive sleepiness in nasal continuous positive airway pressure-treated obstructive sleep apnea/hypopnea syndrome. Sleep. 28, 464-471. evaluated the use of modafinil at doses of 200 or 400 mg/day in patients with OSAH who were already receiving continuous positive airway pressure (CPAP), which is the treatment of choice for overcoming airway obstruction in OSAH. By week 4, modafinil had reduced self-rated daytime sleepiness among participants. By week 12, not only self-rated but also clinician-rated and functional improvements were observed. These benefits were observed regardless of dose.
Another double-blind, placebo-controlled study (N = 157) demonstrated that 400 mg/day of modafinil conferred improvement in self-rated sleepiness within 1 week, and clinician-rated improvement within 4 weeks[19]Pack, AI., Black, JE., Schwartz, JR., Matheson, JK. (2001). Modafinil as adjunct therapy for daytime sleepiness in obstructive sleep apnea. Am J Respir Crit Care Med. 164, 1675-1681.. However, the time taken to fall asleep in a quiet environment remained unchanged.
A 2001 double-blind, placebo-controlled study (N = 32) failed to demonstrate any such benefits[20]Kingshott et al. (2000). Randomized, Double-blind, Placebo-controlled Crossover Trial of Modafinil in the Treatment of Residual Excessive Daytime Sleepiness in the Sleep Apnea/Hypopnea Syndrome. American Journal of Respiratory and Critical Care Medicine Vol. 163, No. 4.. However, with a much smaller sample size than the two aforementioned studies, the power of this study is questionable.
Unregistered medical uses
While there are only three disorders for which the use of modafinil is registered, off-label prescriptions abound. Despite the relative lack of evidence to support its use, and the general reluctance to accept new or unproven treatments among the medical profession, modafinil and, more recently, armodafinil, are achieving ever-increasing popularity. Usage of the drug is likely to continue to rise since the emergence of generic forms of modafinil.
Although modafinil does not necessarily provide greater eugeroic or stimulant effects than other common stimulants such as methylphenidate (Ritalin) or amphetamines, it has far fewer documented side effects, a very low risk of dependence, less stigma, and less stringent prescription requirements. Maybe modafinil won’t be quite as effective as conventional stimulants – but if it’s safer, isn’t it worth a try?
Large-scale double-blind studies assessing the unregistered use of modafinil are relatively rare. However, multiple open-label studies have been conducted. While these studies fail to control for the placebo effect, they often recruit far more participants and therefore have greater power to demonstrate statistically significant results.
As it stands, convincing evidence of the true therapeutic benefits of modafinil exists for only a few conditions for which it is not already registered: attention deficit hyperactivity disorder (ADHD), post-anaesthetic sedation, cocaine addiction, and depression (as an adjunct to antidepressants). However, many anecdotal and small-scale studies have reported potential benefits of modafinil in a wide range of other conditions.
Sleepiness associated with medical conditions
Multiple sclerosis
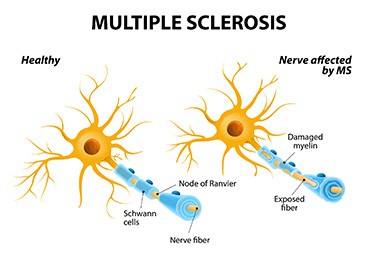
Multiple sclerosis is an immune-mediated disease in which the body’s immune system attacks the nerve cell sheaths in the brain and spinal cord, resulting in chronic, waxing and waning numbness, speech impairment, muscle spasticity and weakness, blurred vision, and often severe fatigue. In one study of MS sufferers, 66% of participants reported that they experienced fatigue almost daily[21]Freal, JE., Kraft, GH., Coryell, JK. (1984). Symptomatic fatigue in multiple sclerosis. Arch Phys Med Rehabil. 65, 135-138.. Overall, while some studies have been suggestive of the utility of modafinil in the treatment of MS, most have shown no real promise.
Evidence regarding the efficacy of modafinil in reducing symptoms of fatigue in MS is mixed. An open-label study (N = 50) showed decreased fatigue and improved functioning among most patients in response to 100 or 200 mg/day of modafinil,[22]Zifko, UA., Rupp, M., Schwarz, S., Zipko, HT., Maida, EM. (2002). Modafinil in treatment of fatigue in multiple sclerosis. Results of an open-label study. J Neurol. 249, 983-987. and a randomised, single-blind, placebo-controlled study (N = 72) showed similar results in response to 200 mg/day of modafinil on several measures of fatigue and sleepiness (interestingly, increasing the dose to 400 mg/day reduced the perception of improvement, which the authors suggested may be due to tolerance or increased side effects).[23]Rammohan, KW., Rosenberg, JH., Lynn, DJ., Blumenfeld, AM., Pollak, CP., Nagaraja, HN. (2002). Efficacy and safety of modafinil (Provigil) for the treatment of fatigue in multiple sclerosis: a two centre phase 2 study. J Neurol Neurosurg Psychiatry. 72, 179-183. However, a larger, double-blind, placebo-controlled trial (N = 115) showed no benefit of modafinil over placebo on three measures of fatigue, with both groups showing similar levels of improvement[24]Stankoff et al. (2005). Modafinil for fatigue in MS: a randomized placebo-controlled double-blind study. Neurology. 64, 1139-1143..
The largest placebo-controlled study conducted to date, in 2011, recruited 121 patients with MS and found no significant difference between modafinil and placebo in terms of fatigue or cognitive function[25]Möller et al. (2011). HAGIL (Hamburg Vigil Study): a randomized placebo-controlled double-blind study with modafinil for treatment of fatigue in patients with multiple sclerosis.
Multiple Sclerosis Journal.. However, the authors report that some results were ‘contradictory’, and suggest that further studies are warranted.
Parkinson’s disease

Parkinson’s disease involves progressive degeneration of the basal ganglia in the brain, which are involved in the coordination of movement, along with reduced levels of dopamine. Symptoms include tremors; muscle rigidity; slow, poorly controlled movement; and, in an estimated 10–50% of cases, sleepiness[26]Rye, D. (2003). Sleepiness and unintended sleep in Parkinson’s disease. Current Treatment Options in Neurology Volume 5, Issue 3. pp 231–239.. Overall, results suggest that, at least for a subset of patients with Parkinson’s disease, modafinil may reduce sleepiness associated with the disease.
A 4-week open-label trial (N = 10),[27]Nieves, AV., Lang, AE. (2002). Treatment of excessive daytime sleepiness in patients with Parkinson’s disease with modafinil. Clin Neuropharmacol. 25, 111-114. a 3-week double-blind, crossover study (N = 21),[28]Adler, CH., Caviness, JN., Hentz, JG., Lind, M., Tiede, J. (2003). Randomized trial of modafinil for treating subjective daytime sleepiness in patients with Parkinson’s disease. Mov Disord. 18, 287-293. and a 2-week double-blind, randomised, placebo-controlled, crossover trial (N = 15)[29]Högl et al. (2002). Modafinil for the treatment of daytime sleepiness in Parkinson’s disease: a double-blind, randomized, crossover, placebo-controlled polygraphic trial. Sleep. 25, 905-909. all showed a reduction in sleepiness among MS patients treated with modafinil. However, a larger 4-week double-blind, placebo-controlled trial (N = 40) of modafinil using the same outcome measures failed to demonstrate a statistically significant difference from the placebo group[30]Ondo, W., Fayle, R., Atassi, F., Jankovic, J. (2005). Modafinil for daytime somnolence in Parkinson’s disease: double blind, placebo controlled parallel trial. J Neurol Neurosurg Psychiatry. 76, 1636–1639.. Even in this study, though, the authors acknowledge that some patients did demonstrate a rapid and significant improvement with modafinil. It may be that some patients stand to experience greater benefits from modafinil treatment than others.
Chronic fatigue syndrome

Chronic fatigue syndrome involves extreme, unrelenting fatigue with no identifiable underlying cause. The mechanism behind it is unknown but viral infections and psychological stress have been implicated. The syndrome may also cause headaches, muscular pain, sleep disturbance, and difficulty focusing. While cognitive behavioural therapy and graded exercise therapy have helped some patients, no reliable treatment has been identified.[31]Whiting, P., Bagnall, AM., Sowden, AJ., Cornell, JE., Mulrow, CD., Ramírez, G. (2001). Interventions for the treatment and management of chronic fatigue syndrome: a systematic review. JAMA. 286, 1360-1368. Evidence to support the use of modafinil for CFS is scant, though online forums suggest that it may be helpful for some[32]Reviews for Modafinil to treat Chronic Fatigue Syndrome.
While a 2004 case report showed promise,[33]Turkington, D., Hedwat, D., Rider, I., Young, AH. (2004). Recovery from chronic fatigue syndrome with modafinil. Hum Psychopharmacol. 19, 63-64. the only randomised, double-blind, placebo-controlled study (N = 14) showed no significant benefit of modafinil compared with placebo in terms of fatigue, attention, mood, or quality of life[34]Randall, DC., Cafferty, FH., Shneerson, JM., Smith, IE., Llewelyn, MB., File, SE. (2005). Chronic treatment with modafinil may not be beneficial in patients with chronic fatigue syndrome. J Psychopharmacol. 19, 647-660.. However, the number of participants was small and the results of this study are therefore unreliable.
Drug-induced sedation

Modafinil has been used in combination with various medications to combat associated sedative effects.
Psychotropics: A double-blind, placebo-controlled trial (N = 24) showed no difference between modafinil and placebo in alleviating psychotropic-associated sedation among schizophrenic patients, though both groups demonstrated improvement in wakefulness and cognition[35]Sevy et al. (2005). Double-blind, placebo-controlled study of modafinil for fatigue and cognition in schizophrenia patients treated with psychotropic medications. J Clin Psychiatry. 66, 839-843.. Conversely, in a series of three case reports, all patients taking modafinil at 200 mg/day along with their regular psychotropic medication experienced increased energy and reduced sleep requirements[36]Ballon, JS., Feifel, D. (2006). A systematic review of modafinil: Potential clinical uses and mechanisms of action. J Clin Psychiatry. 67, 554-566..
Antidepressants: An 8-week placebo-controlled study of modafinil use in patients with major depressive disorder followed up with a 12-week, open-label extension study found that 100–400 mg/day of modafinil was associated with reduced sleepiness and fatigue and improved mood among patients receiving SSRI antidepressant medication[37]Thase, M., Fava, M., DeBattista, C., Arora, S., Hughes, R. (2014). Modafinil Augmentation of SSRI Therapy in Patients with Major Depressive Disorder and Excessive Sleepiness and Fatigue: A 12-Week, Open-label, Extension Study. CNS Spectrums Volume 11, Issue 2. pp. 93-102..
Anaesthetics: In a double-blind, placebo-controlled study (N = 34), patients who took 200 mg of modafinil upon awakening from anaesthesia reported significantly less fatigue over the following 24 hours than those who received placebo[38]Larijani, GE., Goldberg, ME., Hojat, M., Khaleghi, B., Dunn, JB., Marr, AT. (2004). Modafinil improves recovery after general anesthesia. Anesth Analg. 98, 976-981.. This is an important result, as it means that modafinil can be used to improve the alertness of outpatients upon discharge and reduce the chance of anaesthesia-related complications while outside the care of the hospital.
Opioids: In a retrospective study of patients experiencing sedation secondary to opioid medication (N = 11), modafinil at 200 to 600 mg/day was associated with significant reductions in sedation[39]Webster, L., Andrews, M., Stoddard, G. (2003). Modafinil treatment of opioid-induced sedation. Pain Med. 4, 135-140.. These results are promising, but the low power of the study means they must be interpreted with caution.
Other conditions associated with fatigue
- Polio: A single double-blind, placebo-controlled, crossover trial (N = 14) for treatment of sedation in patients with a prior diagnosis of polio showed no significant benefit to modafinil treatment [40]Chan, KM., Strohschein, FJ., Rydz, D., Allidina, A., Shuaib, A., Westbury, CF. (2006). Randomized controlled trial of modafinil for the treatment of fatigue in postpolio patients. Muscle Nerve. 33, 138-141..
- HIV: In an open-label study (N = 30), 80% of subjects self-reported reduced fatigue with modafinil treatment, and those with comorbid depression also experienced an improvement in depression scores.[41]Rabkin, JG., McElhiney, MC., Rabkin, R., Ferrando, SJ. (2004). Modafinil treatment for fatigue in HIV+ patients: a pilot study. The Journal of Clinical Psychiatry. 65, 1688-1695. However, this study did not include a placebo group.
- Dementia: An open-label study (N = 8)[42]Howcroft, DJ., Jones, RW. (2005). Does modafinil have the potential to improve disrupted sleep patterns in patients with dementia? International Journal of Geriatric Psychiatry. 20, 492-495. and a retrospective study (N = 5)[43]Cochran, J. (2011). Effect of Modafinil on Fatigue Associated with Neurological Illnesses. Journal of Chronic Fatigue Syndrome Volume 8, 2000 – Issue 2. Pages 65-70. concluded that modafinil may improve symptoms of fatigue in dementia, but neither was conclusive.
- Fibromyalgia: Modafinil at 150 to 300 mg/day was associated with a moderate reduction in fatigue and improvement in alertness in two of four patients with chronic fibromyalgia, a chronic condition causing muscular pain and disturbances to mood and sleep.[44]Schaller, JL., Behar, D. (2001). Modafinil in fibromyalgia treatment. J Neuropsychiatry Clin Neurosci. 13, 530-531.
- Case reports have shown that modafinil may be helpful in alleviating fatigue due to primary biliary cirrhosis,[45]Kaplan, MM., Bonis, PA. (2005). Modafinil for the treatment of fatigue in primary biliary cirrhosis. Ann Intern Med. 143, 546-547. amyotrophic lateral sclerosis,[46]Carter et al. (2005). Modafinil to treat fatigue in amyotrophic lateral sclerosis: an open label pilot study. Am J Hosp Palliat Care. 22, 55-59. and myasthenia gravis.[47]Lechin et al. (2000). Enhancement of noradrenergic neural transmission: an effective therapy of myasthenia gravis: a report on 52 consecutive patients. J Med. 31, 333-361.
- Chronic traumatic brain injury: A double-blind, placebo-controlled trial showed no clear benefit to modafinil treatment in 12 measures of fatigue and sleepiness.[48]Jha et al. (2008). A Randomized Trial of Modafinil for the Treatment of Fatigue and Excessive Daytime Sleepiness in Individuals with Chronic Traumatic Brain Injury. The Journal of Head Trauma Rehabilitation: January-February 2008 – Volume 23 – Issue 1. p 52–63.
Non-wakefulness-related applications
Attention deficit hyperactivity disorder (ADHD)

ADHD is characterised by chronic, persistent difficulties with attention and focus; hyperactivity and impulsivity; or both. It is thought to affect approximately 7% of children[49]Willcutt, E. (2012). The Prevalence of DSM-IV Attention-Deficit/Hyperactivity Disorder: A Meta-Analytic Review. Neurotherapeutics. 9, 490–499. and approximately 3% of adults,[50]Fayyad et al. (2007). Cross-national prevalence and correlates of adult attention-deficit hyperactivity disorder. Br J Psychiatry. 190, 402-409. though estimates vary. Methylphenidate (Ritalin) is the most commonly used medical treatment for ADHD, followed by different forms of amphetamines.[51]Attention deficit hyperactivity disorder (ADHD): Overview. (2015). Informed Health Online [Internet].
A double-blind, crossover, placebo-controlled study (N = 22) of the efficacy of dextroamphetamine and modafinil showed that both active medications were equally effective in treating adult ADHD.[52]Taylor, FB., Russo, J. (2000). Efficacy of modafinil compared to dextroamphetamine for the treatment of attention deficit hyperactivity disorder in adults. J Child Adolesc Psychopharmacol. 10, 311-320. In another double-blind, randomised, placebo-controlled crossover study (N = 20), a single 200 mg dose of modafinil was shown to improve short-term memory span, visual memory, spatial planning, and stop-signal motor inhibition in adults both with and without ADHD relative to placebo.[53]Turner, DC., Clark, L., Dowson, J., Robbins, TW., Sahakian, BJ. (2004). Modafinil improves cognition and response inhibition in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 55, 1031-1040. These results are similar to those obtained with methylphenidate, but without associated side effects – though the authors note that improvements with a single dose do not necessarily correlate to long-term improvements.
However, Cephalon, the manufacturer of modafinil, conducted a larger, double-blind, placebo-controlled trial (N = 113) examining the efficacy of 100 or 400 mg/day of modafinil in adults with ADHD and failed to show a statistically significant benefit on the DSM-IV ADHD Behavior Checklist for Adults.[54]Cephalon Reports No Benefit from PROVIGIL in Study of Adults with ADHD. (2000). News Release.
A randomised, placebo-controlled trial (N = 24) of an average of 200 mg/day of modafinil in children with ADHD showed a statistically significant improvement in attention and parent and teacher ratings, albeit no significant improvement on the ADHD Rating Scale-IV.[55]Rugino, TA., Samsock, TC. (2003). Modafinil in children with attention-deficit hyperactivity disorder. Pediatr Neurol. 29, 136-142. Reinforcing these findings, a large, multi-centre, double-blind, placebo-controlled trial (N = 248) found that modafinil (at varying doses of 170 to 425 mg/day titrated based on tolerability and efficacy) conferred statistically significant improvements on the ADHD Rating Scale-IV Home and School Versions and the Conners’ Parent and Teacher Rating Scales over 9 weeks in children with ADHD.[56]Biederman J, Swanson JM, Wigal SB, et al. (2006). Modafinil improves symptoms of ADHD compared with placebo in young people. Evid Based Mental Health. 9, 68.
But despite the promising results of this large-scale study, modafinil is still not recommended in children. The primary reason for this is that, while the efficacy of modafinil is not debated, the FDA has determined that the risk of potentially fatal adverse skin reactions is too serious for the drug to be considered safe for administration to millions of children. This decision was based primarily on one particular trial conducted by Cephalon in which a single child developed Stevens-Johnson syndrome, a rare and potentially fatal disorder characterised by peeling and blistering over the entire body, which continued to progress for days after discontinuation of the drug.[57]Hamilton, J. (2006). FDA Committee Rejects ADHD use for Modafinil. Heard on NPR Morning Edition.
Depression

Insomnia is common in depression, frequently resulting in fatigue.[58]Fava, M. (2004). Daytime sleepiness and insomnia as correlates of depression. J Clin Psychiatry. 65 Suppl 16:27-32. Studies suggest that modafinil may be a useful adjunct therapy in alleviating this aspect of depression, especially since the incidence of inadequate response to traditional antidepressants is high. However, results suggest that the usefulness of modafinil in the treatment of depression may extend beyond its effects on wakefulness.
In a 2010 case report, seven patients with significant fatigue who received modafinil in addition to an SSRI antidepressant experienced not only resolution of this symptom but also an improvement on both the cognitive and physical domains of the Hamilton Depression Rating Scale within 2 weeks.[59]Menza, MA., Kaufman, KR., Castellanos, A. (2000). Modafinil augmentation of antidepressant treatment in depression. J Clin Psychiatry. 61, 378-381.
A 2013 study reviewed six randomised controlled trials (N = 910) of the use of modafinil in people with major depressive disorder (N = 568) or bipolar depression (N = 342) who were already receiving antidepressant therapy.[60]Goss, AJ., Kaser, M., Costafreda, SG., Sahakian, BJ., Fu, CHY. (2013). Modafinil Augmentation Therapy in Unipolar and Bipolar Depression: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Early Career Psychiatrists Meta-Analysis. The study revealed significant effects of modafinil on improvements in fatigue, overall depression scores and remission rates in both types of depression.
One of the studies that the review examined (N = 314) found that among patients with major depressive disorder who had not responded adequately to treatment with an SSRI antidepressant, the addition of 200 mg/day of modafinil was associated with greater improvements in severely affected individuals than in more mildly affected individuals.
In one case report[61]Kaufman, KR., Menza, MA., Fitzsimmons, A. (2002). Modafinil monotherapy in depression. Eur Psychiatry. 17, 167-169. and one retrospective study (N = 45),[62]Price, CS., Taylor, FB. (2005). A retrospective chart review of the effects of modafinil on depression as monotherapy and as adjunctive therapy. Depress Anxiety. 21, 149-153. modafinil monotherapy has been associated with improvements on multiple depression rating scores. However, without a placebo group, it is impossible to know whether these are true effects. Another case report describes improvement in two individuals with bipolar affective disorder who continued to experience excessive sleepiness despite the resolution of other symptoms. However, the study did not evaluate long-term effects.[63]Fernandes, PP., Petty, F. (2003). Modafinil for remitted bipolar depression with hypersomnia. Ann Pharmacother. 37, 1807-1809.
Yet another double-blind, randomised, placebo-controlled study (N = 60) has looked at the potential role of modafinil in ameliorating persistent cognitive dysfunction among individuals who have recovered from symptoms relating to mood.[64]Kaser et al. (2017). Modafinil Improves Episodic Memory and Working Memory Cognition in Patients With Remitted Depression: A Double-Blind, Randomized, Placebo-Controlled Study. Biol Psychiatry Cogn Neurosci Neuroimaging. 2, 115–122. Participants receiving a single 200 mg dose of modafinil were found to have significantly better performance in terms of both episodic memory (memory of events) and working memory (storage of information for immediate processing), though there was no improvement in planning or sustained attention.
Cocaine dependence

A 2003 study (N = 7) evaluated the use of modafinil in the treatment of cocaine addicts and found that it reduced feelings of euphoria associated with cocaine without contributing to any additional side effects.[65]Dackis, C., O’Brien, C. (2003). Glutamatergic agents for cocaine dependence. Ann N Y Acad Sci. 1003, 328-345. In 2005, a double-blind, placebo-controlled trial (N = 62) followed cocaine-dependent patients for 8 weeks. All patients received cognitive behavioural therapy, and either 400 mg/day of modafinil or a placebo. The treatment group demonstrated a statistically significant decrease in positive urine samples for a cocaine metabolite and a significantly longer period of abstinence from cocaine, though withdrawal symptoms did not appear to be reduced.[66]Dackis, CA., Kampman, KM., Lynch, KG., Pettinati, HM., O’Brien, CP. (2005). A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology. 30, 205-211.
Since then, two randomised, double-blind, placebo-controlled studies of modafinil have been conducted with much larger sample sizes. The first, in 2009 (N = 210), showed no influence of modafinil on the number of days of abstinence over 12 weeks, though the improvement was documented in the maximum number of consecutive days of abstinence and perception of cravings.[67]Anderson et al. (2009). Modafinil for the treatment of cocaine dependence. Drug and Alcohol Dependence Volume 104, Issues 1-2, Pages 133–139. The second, in 2012 (N = 210), combined modafinil with weekly cognitive behavioural therapy and found no significant difference from placebo on measures of abstinence, cravings or withdrawal.[68]Dackis et al. (2012). A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Journal of Substance Abuse Treatment Volume 43, Issue 3, Pages 303–312.
Schizophrenia

Conventional stimulants are associated with the potential risk of worsening psychosis in patients with psychotic disorders. But the same may not be true of modafinil.
In an open-label study (N = 11) of schizophrenics, 4 weeks of modafinil treatment at 100–200 mg/day, in addition to the antipsychotic regimen, was associated with improvement in clinical condition, global functioning, and cognition, with 89% of patients considering themselves to be clinically improved.[69]Rosenthal, MH., Bryant, SL. (2004). Benefits of adjunct modafinil in an open-label, pilot study in patients with schizophrenia. Clin Neuropharmacol. 27, 38-43. In a double-blind, placebo-controlled, crossover study (N = 20), the addition of modafinil at up to 200 mg/day significantly improved shifting of attention from one focus to another, which is known to be deficient in schizophrenic patients.[70]Rosenthal, MH., Bryant, SL. (2004). Benefits of adjunct modafinil in an open-label, pilot study in patients with schizophrenia. Clin Neuropharmacol. 27, 38-43.
However, two double-blind, placebo-controlled trials (N = 24[71]Sevy et al. (2005). Double-blind, placebo-controlled study of modafinil for fatigue and cognition in schizophrenia patients treated with psychotropic medications. The Journal of Clinical Psychiatry. 66, 839-843. and N = 20)[72]Pierre, JM., Peloian, JH., Wirshing, DA., Wirshing, WC., Marder, SR. (2007). A randomized, double-blind, placebo-controlled trial of modafinil for negative symptoms in schizophrenia. The Journal of Clinical Psychiatry. 68, 705-710. have failed to show a statistically significant positive effect of modafinil on fatigue, cognition, or symptoms over 8 weeks. One subject in one of these studies experienced increased psychosis, though it is unknown whether this was a result of modafinil administration. One other case report describes increased psychosis concurrent with modafinil treatment, though the patient’s condition was severe and the dose of modafinil was high.[73]Narendran et al. (2002). Is Psychosis Exacerbated by Modafinil? Arch Gen Psychiatry. 59, 292-293.
A 2012 review of the literature on the use of modafinil in schizophrenia suggested that modafinil may facilitate cognitive function, including memory and problem-solving, as well as emotional processing.[74]Scoriels, L., Jones, P., Sahakian, B. (2012). Modafinil effects on cognition and emotion in schizophrenia and its neurochemical modulation in the brain. Neuropharmacology Volume 64. Pages 168-184. However, a 2015 meta-analysis was less promising. This study pooled the results of eight randomised, controlled trials (total N = 372) of the use of modafinil or armodafinil in addition to antipsychotics in schizophrenia.[75]Andrade, C., Kisely, S., Monteiro, I., Rao S. (2015). Antipsychotic augmentation with modafinil or armodafinil for negative symptoms of schizophrenia: systematic review and meta-analysis of randomized controlled trials. J Psychiatr Res. 60, 14-21. Modafinil at 200 mg/day slightly but significantly attenuated negative symptom ratings and total psychopathology ratings but did not influence positive symptom ratings. However, when acutely ill patients were excluded, this treatment effect disappeared, suggesting that chronically ill patients may not stand to gain from modafinil treatment. No significant difference was observed compared with the placebo in measures of cognition, fatigue, daytime drowsiness, or adverse events.
Obesity

Amphetamines are known to have appetite-suppressant qualities, but their side effects limit their use in this context.[76]Bray, G. (1993). Use and Abuse of Appetite-Suppressant Drugs in the Treatment of Obesity. Ann Intern Med. 119, 707-713. Modafinil may offer a viable alternative, though the evidence is weak. In a double-blind study (N = 11), participants taking 200 mg/day of modafinil ate less overall, but the low number of participants made it hard to conclude.
In a case report of a schizophrenic patient with significant weight gain due to clozapine who had previously been refractory to weight-loss techniques, modafinil was cited as an important factor in the onset of weight loss despite the continuation of clozapine.[77]Henderson et al. (2005). Modafinil-associated weight loss in a clozapine-treated schizoaffective disorder patient. Ann Clin Psychiatry. 17, 95-97. However, a double-blind, placebo-controlled trial found no statistically significant effect of 300 mg/day modafinil on weight in patients receiving clozapine.[78]Henderson et al. (2011). Effects of modafinil on weight, glucose and lipid metabolism in clozapine-treated patients with schizophrenia. Schizophrenia Research Volume 130, Issues 1-3. Pages 53–56.
Cerebral palsy

In a 2006 retrospective review of children with spastic cerebral palsy (N = 120), 29 of 59 patients in the modafinil treatment group showed improvements in gait, with six patients gaining the ability to walk unassisted.[79]Hurst, DL., Lajara-Nanson, WA., Lance-Fish, ME. (2006). Walking With Modafinil and Its Use in Diplegic Cerebral Palsy. Journal of Child Neurology Vol 21, Issue 4. Over the study period, only three of the patients in the placebo group demonstrated improvements in gait, and these were not nearly as dramatic as those observed in the treatment group. This is compelling evidence of the usefulness of modafinil for improving muscle tone in cerebral palsy patients, an effect that is thought to involve modulation of the mechanisms of spasticity in the brainstem.[80]Hurst, DL., Lajara-Nanson, W. (2002). Use of Modafinil in Spastic Cerebral Palsy. Journal of Child Neurology Vol 17, Issue 3. A case study also reports the resolution of chronic drooling due to swallowing dysfunction in two cerebral palsy patients treated with modafinil, along with improvements in coordination and speech.[81]Hurst, D., Cedrone, N. (2006). Modafinil for Drooling in Cerebral Palsy. Journal of Child Neurology Vol 21, Issue 2.
Addiction and impulsivity

Research on the effects of modafinil in people with addiction remains limited, but it is an interesting area given the reduction in impulsivity that has been documented in people with ADHD. A 2013 randomised, double-blind, placebo-controlled study (N = 83) of alcohol-dependent patients demonstrated reduced self-reported impulsivity, but showed no effect on behavioural measures of impulsivity or alcohol consumption.[82]Joos et al. (2013). Effect of modafinil on impulsivity and relapse in alcohol dependent patients: a randomized, placebo-controlled trial. Eur Neuropsychopharmacol. 23, 948-955. However, among the subgroup who had poor impulse control, to begin with, there was a longer time to relapse and increased percentage of abstinent days. Concerningly, among those with better baseline inhibition, the percentage of abstinent days decreased and the percentage of heavy drinking days increased. We must be very cautious about using modafinil in this application.
In the context of pathological gambling, a small-scale (N= 20) placebo-controlled, double-blind study also found varying effects of modafinil depending on baseline impulsivity.[83]Zack, M., Poulos, CX. (2008). Effects of the atypical stimulant modafinil on a brief gambling episode in pathological gamblers with high vs. low impulsivity. Journal of Psychopharmacology Vol 23, Issue 6. Those with high baseline impulsivity experienced a reduced desire to gamble and improved decision-making, whereas those with low baseline impulsivity experienced the opposite effect. Note that this is the opposite trend to that observed in the study of alcoholics! Again, much research is required before modafinil can be recommended in this context.
Social phobia
A single case report describes improvement in symptoms of social phobia and amphetamine dependence.[84]Ballon, JS., Feifel, D. (2006). A systematic review of modafinil: Potential clinical uses and mechanisms of action. J Clin Psychiatry. 67, 554-566.
Nootropic effects

While the majority of studies examining the cognitive-enhancing properties of modafinil have been conducted among people with conditions affecting sleep or cognition, it is starting to receive more attention in the context of use in healthy individuals.
In 2015, a systematic review was published that examined 24 randomised, placebo-controlled studies evaluating various aspects of cognitive function in healthy, non-sleep-deprived participants receiving modafinil.[85]Battleday, RM., Brem, A.-K. (2015). Modafinil for cognitive neuroenhancement in healthy non-sleep-deprived subjects: A systematic review. European Neuropsychopharmacology Volume 25, Issue 11. Pages 1865–1881. The study spanned 24 years.
Some of the studies examined in this review looked at participants’ performance on simple tasks that assessed basic cognitive functioning and generally focused on one distinct aspect of cognition at a time. While these tests may be useful to distinguish between people with normal versus abnormal cognition, they may not distinguish between normal versus heightened cognition and are difficult to extrapolate to everyday functioning, where we must integrate multiple aspects of cognition simultaneously. Sure enough, the review failed to find a consistent benefit to modafinil versus placebo on simple tasks.
However, when it came to those studies measuring more complex aspects of cognition, it was a very different story. Consistent improvement was documented in multiple aspects of cognition, including attention, the ability to focus on a task and process relevant information; learning and memory; and executive function, which includes the ability to take in information and use it to come up with plans or strategies.
Attention

Attention refers to the ability to distinguish between relevant and irrelevant information and allocate cognitive resources to relevant information to process it efficiently. Most studies have shown no statistically significant improvement in attention in subjects that took modafinil, though two studies (N = 18[86]Baranski, JV., Pigeau, R., Dinich, P., Jacobs, I. (2004). Effects of modafinil on cognitive and meta‐cognitive performance. Human Psychopharmacology: Clinical and Experimental Volume 19, Issue 5. Pages 323-332. and N = 60)[87]Randall et al. (2005). Does Modafinil Enhance Cognitive Performance in Young Volunteers Who Are Not Sleep-Deprived? Journal of Clinical Psychopharmacology. 25, 175-179. did document the improved ability to sustain attention.
Executive function

Executive function involves using the information to plan and integrate cognitive processes and behaviour to achieve goals. This consists of three main domains: inhibitory control, the ability to inhibit irrelevant information; cognitive flexibility, the ability to shift attention based on changing task demands; and working memory, the ability to retain information while working on it. These three domains in combination then give rise to higher-order decision-making and abstract thinking.
In one study (N = 60), modafinil significantly enhanced performance on tests of digit span (how many numbers a person can remember), visual pattern recognition, spatial planning and stop-signal reaction time.[88]Turner et al. (2003). Cognitive enhancing effects of modafinil in healthy volunteers. Psychopharmacology Volume 165, Issue 3. pp 260–269. Another study (N = 44) demonstrated improved inhibitory control among participants taking modafinil.
One study (N = 38) used functional MRI to demonstrate changes in activity in certain areas of the brain suggestive of enhanced executive function following modafinil administration,[89]Rasetti et al. (2010). Modulatory Effects of Modafinil on Neural Circuits Regulating Emotion and Cognition. Neuropsychopharmacology volume 35. pages 2101–2109. while another study showed that modafinil was associated with increases in electrical activity in the brain during tasks involving executive function.[90]Minzenberg et al. (2014). Modafinil augments oscillatory power in middle frequencies during rule selection. Psychophysiology Volume 51, Issue 6.
When it comes to working memory, results are mixed – as many studies have yielded results suggestive of benefits as those that have failed to show such an association. For cognitive flexibility, no benefits have been documented and one study was even suggestive of a deleterious effect.[91]Randall, DC., Fleck, NL., Shneerson, JM., File, SE. (2004). The cognitive-enhancing properties of modafinil are limited in non-sleep-deprived middle-aged volunteers. Pharmacology Biochemistry and Behavior Volume 77, Issue 3. Pages 547-555. Similar to findings on working memory, research on the influence of modafinil on short-term memory has been mixed. Benefits to learning and memory in the short term have been found in some studies, but just as many have failed to corroborate these findings.
Higher-order decision-making and abstract thinking

While results of studies examining individual aspects of executive function have not always revealed consistent results, it may be that the true benefits of modafinil lie not in any specific cognitive domains but in the integration of multiple domains.
Two large studies have shown improved performance on difficult tasks in subjects treated with modafinil,[92]Turner et al. (2003). Cognitive enhancing effects of modafinil in healthy volunteers. Psychopharmacology Volume 165, Issue 3. pp 260–269. and one also found that participants who received modafinil enjoyed the tasks more.[93]Müller et al. (2013). Effects of modafinil on non-verbal cognition, task enjoyment and creative thinking in healthy volunteers. Neuropharmacology Volume 64. Pages 490-495.
Two studies demonstrated a positive effect of modafinil on fluid intelligence – how well people can think abstractly and respond to tasks by thinking laterally and coming up with novel solutions.[94]Baranski, JV., Pigeau, R., Dinich, P., Jacobs, I. (2004). Effects of modafinil on cognitive and meta‐cognitive performance. Human Psychopharmacology: Clinical and Experimental Volume 19, Issue 5. Pages 323-332.,[95]Baranski, JV., Pigeau, R., Dinich, P., Jacobs, I. (2004). Effects of modafinil on cognitive and meta‐cognitive performance. Human Psychopharmacology: Clinical and Experimental Volume 19, Issue 5. Pages 323-332.
Still, other studies have looked at quite specific combinations of cognitive functions.
- One study found improved accuracy on a task that required shifting of attention under demanding conditions, though this benefit was not seen when attention shifting was required infrequently throughout an ongoing task.[96]Marchant et al. (2009). Modafinil improves rapid shifts of attention. Psychopharmacology Volume 202, Issue 1–3. pp 487–495.
- Another study found that modafinil conferred increased alertness and improved implicit (that is, non-intentional) learning, in terms of both accuracy and reaction time.[97]Pringle, A., Browning, M., Parsons, E., Cowen, PJ., Harmer, CJ. (2013). Early markers of cognitive enhancement: developing an implicit measure of cognitive performance. Psychopharmacology Volume 230, Issue 4. pp 631–638.
- Other research (n = 33) in which participants were trained in a new language found that those who were receiving modafinil acquired the new language more effectively.[98]Gilleen et al. (2014). Modafinil combined with cognitive training is associated with improved learning in healthy volunteers – A randomised controlled trial. European Neuropsychopharmacology Volume 24, Issue 4. Pages 529–539.
- Still another study showed that modafinil enhances visual short-term memory capacity and speed of processing of information, which correlated with participants’ self-ratings of alertness, among people whose baseline performance was poor.[99]Finke et al. (2010). Effects of modafinil and methylphenidate on visual attention capacity: a TVA-based study. Psychopharmacology Volume 210, Issue 3. pp 317–329.
Creativity

Overall, results of studies assessing the effect of modafinil on creativity – defined as the ability to produce ideas that are both novel and meaningful[100]Dietrich, A. (2004). The cognitive neuroscience of creativity. Psychonomic Bulletin & Review December 2004, Volume 11, Issue 6. pp 1011–1026. – have found no beneficial effect. One study even demonstrated reduced performance on divergent thinking (the ability to find multiple solutions to a problem) with modafinil.[101]Mohamed, AD., Lewis, CR. (2014). Modafinil Increases the Latency of Response in the Hayling Sentence Completion Test in Healthy Volunteers: A Randomised Controlled Trial. PLoS ONE. However, the same study found that among those who were determined based on a questionnaire to have low baseline creativity, scores of creativity did significantly improve. So it may be that modafinil has a role in increasing creativity only in individuals who are not very creative to begin with.
Mood

While studies in people with disorders or diseases have tended to report improvements in mood, these effects do not appear to extrapolate to healthy users, with most studies of healthy people revealing no changes in this regard. One study reported increased anxiety among some participants.[102]Randall, DC., Shneerson, JM., Plaha, KK., File, SE. (2002). Modafinil affects mood, but not cognitive function, in healthy young volunteers. Human Psychopharmacology: Clinical and Experimental Volume 18, Issue 3. Pages 163-173.
Physical performance enhancement

A small study (N = 15) showed that modafinil prolongs exercise time to exhaustion and also reduces the perception of effort required to maintain this threshold, which has led to its use as a performance-enhancing agent in elite sport.[103]Jacobs, I., Bell, DG. (2004). Effects of acute modafinil ingestion on exercise time to exhaustion. Med Sci Sports Exerc. 36, 1078-1082. It has been on the World Anti-Doping Agency’s ‘Prohibited List’ since 2004.[104]List of Prohibited Substances and Methods (2018). World Anti-Doping Agency.
Neurological health
In addition to enhancement of cognitive function, studies suggest that modafinil may improve neurological health via an antioxidant effect, neutralising damaging free radicals in the brain.[105]Gerrard, P., Malcolm, R. (2007). Mechanisms of modafinil: A review of current research. Neuropsychiatr Dis Treat. 3, 349–364. One study also found that modafinil attenuates glutamate-induced excitotoxicity in cortical neurons.[106]Antonelli et al. (1998). Modafinil prevents glutamate cytotoxicity in cultured cortical neurons. NeuroReport Volume 9 – Issue 18. p 4209–4213.
The body of evidence to support the cognitive-enhancing effect of modafinil is continuing to expand, and it certainly does appear to confer benefits at least for some aspects of cognition. However, studies to date have utilised a range of methodologies and approaches, making it difficult to synthesise the results to draw strong conclusions. A large, placebo-controlled study using an appropriate study framework will be necessary to further elucidate the benefits of modafinil for healthy people.
Mechanism of action
Despite the growing number of studies evaluating the use of modafinil in a variety of contexts, scientists still have not elucidated the exact mechanisms by which modafinil works. What we do know is that modafinil works via multiple distinct mechanisms that are not shared by conventional stimulants such as methylphenidate, methamphetamine and cocaine.[107]Simon, P., Hémet, C., Ramassamy, C., Costentin, J. (1995). Non-amphetaminic mechanism of stimulant locomotor effect of modafinil in mice. Eur Neuropsychopharmacol. 5, 509-514.
Modafinil has effects on many different neurotransmitters in the brain, including histamine, dopamine, adrenaline, noradrenaline, gamma-aminobutyric acid (GABA), glutamate, serotonin, and orexin (also known as hypocretin). Many of these effects are direct, but there is also a complex interplay between neurotransmitters in the brain, with alterations in the level of one neurotransmitter often entailing changes in the levels of many others.
Modafinil seems to specifically alter activity and transmission within and between particular areas of the brain.[108]Ellis et al. (1999). Functional magnetic resonance imaging neuroactivation studies in normal subjects and subjects with the narcoleptic syndrome. Actions of modafinil. J Sleep Res. 8, 85-93. This potentiates more specific effects on cognition and avoids the diffuse brain stimulation associated with other stimulants. For example, modafinil has been found to increase activity in an area of the brain known as the Dorsal Attention Network, which is thought to aid in differentiating relevant and non-relevant stimuli, and improve connectivity in a part of the Frontal Parietal Control network, which is thought to be involved with planning.[109]Esposito et al. (2013). Acute Effects of Modafinil on Brain Resting State Networks in Young Healthy Subjects. PLoS ONE.
Dopamine
Dopamine is a neurotransmitter involved with the regulation of mood, motivation, and focus. A 2009 study examined the effects of modafinil on a range of dopamine receptors and transporters in vitro and found significant activity only at dopamine transporters (DATs), where it acts as a dopamine reuptake inhibitor.[110]Zolkowska et al. (2009). Evidence for the Involvement of Dopamine Transporters in Behavioral Stimulant Effects of Modafinil. J Pharmacol Exp Ther. 329, 738–746. This is the same site of action as cocaine and methylphenidate, but the type of binding is different,[111]Federici et al. (2013). Electrophysiological and amperometric evidence that modafinil blocks the dopamine uptake transporter to induce behavioral activation. Neuroscience. 252, 118-124. and this corresponds to a different profile of effects.[112]Reith et al. (2015). Behavioral, biological, and chemical perspectives on atypical agents targeting the dopamine transporter. Drug Alcohol Depend. 147, 1-19.
But is this a significant contributor to modafinil’s effects? It seems likely. A human positron emission tomography (PET) study confirmed that modafinil significantly increases extracellular dopamine activity in the brain.[113]Volkow et al. (2009). Effects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implications. JAMA. 301, 1148-1154. Administration of dopamine receptor antagonists to mice has been shown to attenuate modafinil’s wakefulness-promoting effects,[114]Qu, WM., Huang, ZL., Xu, XH., Matsumoto, N., Urade, Y. (2008). Dopaminergic D1 and D2 receptors are essential for the arousal effect of modafinil. J Neurosci. 28, 8462-8469.,[115]Quisenberry, AJ., Baker, LE. (2015). Dopaminergic mediation of the discriminative stimulus functions of modafinil in rats. Psychopharmacology (Berl). 232, 4411-4419. while blocking both of the two types of dopamine receptors eliminates these effects altogether.[116]Wisor, J. (2013). Modafinil as a catecholaminergic agent: empirical evidence and unanswered questions. Front Neurol. 4, 139.
However, interestingly, one study comparing modafinil analogues found no difference in wakefulness-promoting effects among analogues with different affinities for the DAT receptor, and some analogues with very poor affinity still effectively promoted wakefulness.[117]Dunn et al. (2012). Wake promoting agents: search for next generation modafinil, lessons learned: part III. Bioorg Med Chem Lett. 22, 3751-3753.
This is but a sample of the research findings with regard to modafinil’s effects on dopamine in the brain, but if nothing else it shows that the mechanisms by which modafinil alters dopamine balance in the brain are complex and selective.
This is in contrast to amphetamines and other stimulants, which directly stimulate dopamine release and increase dopamine levels rapidly and markedly. Modafinil works more by blocking the receptors that remove dopamine from the synapses, gradually elevating the amount of dopamine available in the brain. This reduces the euphoric ‘high’ that can lead to addiction.
Another important finding to support the role of dopamine in modafinil’s stimulation of wakefulness effects is that it appears not to work in individuals with a recessive genetic condition that reduces the activity of the enzyme catechol-O-methyl transferase (COMT), which is involved in the breakdown of dopamine in the brain.[118]Bodenmann et al. (2009). Pharmacogenetics of modafinil after sleep loss: catechol-O-methyltransferase genotype modulates waking functions but not recovery sleep. Clin Pharmacol Ther. 85, 296-304. Presumably, this mutation precludes the efficacy of dopamine reuptake inhibitors because there is already impaired dopamine reuptake in the brain.
Noradrenaline
As early as 1990, researchers were looking into the effect of modafinil on noradrenaline. Modafinil was found to result in an increase in locomotor activity in rats and monkeys, but this effect was not observed in those that had also received antagonists of ‘alpha-1 adrenoceptors’ – receptors that respond to noradrenaline.[119]Duteil et al. (1990). Central alpha 1-adrenergic stimulation in relation to the behaviour stimulating effect of modafinil; studies with experimental animals. Eur J Pharmacol. 180, 49-58. This is highly suggestive of a role of noradrenaline in producing the locomotor activity increases seen with modafinil. Another study in mice has since shown very similar results.[120]Stone, EA., Cotecchia, S., Lin, Y., Quartermain, D. (2002). Role of brain α1B‐adrenoceptors in modafinil‐induced behavioral activity. Synapse Volume 46, Issue 4. A 2006 study further supported this hypothesis, showing that modafinil binds not only to DATs but also to noradrenaline transporters.[121]Madras et al. (2006). Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. J Pharmacol Exp Ther. 319, 561-569.
However, another study found that while drugs that stimulate adrenoceptors effectively treat cataplexy in dogs, modafinil does not, suggesting that the effect of modafinil on these receptors may not be very strong[122]Shelton, J., Nishino, S., Vaught, J., Dement, WC., Mignot, E. (1995). Comparative effects of modafinil and amphetamine on daytime sleepiness and cataplexy of narcoleptic dogs. Sleep. 18, 817-826. – though another proposed explanation is that this apparent discrepancy is due to the anatomical specificity of modafinil.[123]Wisor, JP., Eriksson, KS. (2005). Dopaminergic—adrenergic interactions in the wake promoting mechanism of modafinil. Neuroscience Volume 132, Issue 4. Pages 1027-1034.
Orexin and histamine
Modafinil strongly activates orexin neurons in the lateral hypothalamus.[124]Scammell et al. (2000). Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J Neurosci. 20, 8620-8628. Orexin, also referred to as hypocretin, is a neurotransmitter produced in the hypothalamus that is involved in the regulation of appetite[125]Rodgers, RJ., Ishii, Y., Halford, JC., Blundell, JE. (2002). Orexins and appetite regulation. Neuropeptides. 36, 303-325. and wakefulness.[126]Lin et al. (1999). The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 98, 365-376. Orexin stimulates glutaminergic nerve firing in the hypothalamic circuits associated with arousal regulation.[127]Li, Y., Gao, XB., Sakurai, T., van den Pol, AN. (2002). Hypocretin/Orexin excites hypocretin neurons via a local glutamate neuron-A potential mechanism for orchestrating the hypothalamic arousal system. Neuron. 36, 1169-1181.
Orexin also stimulates the release of histamine in the hypothalamus, influencing arousal and regulating the sleep-wake cycle.[128]Ko, EM., Estabrooke, IV., McCarthy, M., Scammell, TE. (2003). Wake-related activity of tuberomammillary neurons in rats. Brain Research. 992, 220-226. While peripheral administration of modafinil to rats elevates histamine levels, this elevation does not occur when modafinil is administered directly into the hypothalamus, suggesting that the increase in histamine associated with modafinil is a result of its effects on the orexin system.[129]Ishizuka, T., Sakamoto, Y., Sakurai, T., Yamatodani, A. (2003). Modafinil increases histamine release in the anterior hypothalamus of rats. Neurosci Lett. 339, 143-146.
In a study of mice, the administration of an agent that depletes histamine in the brain blocked the effect of modafinil on locomotor activity.[130]Ishizuka, T., Murakami, M., Yamatodani, A. (2008). Involvement of central histaminergic systems in modafinil-induced but not methylphenidate-induced increases in locomotor activity in rats. Eur J Pharmacol. 578, 209-215. The fact that a similar effect was observed in rats given alpha-1 adrenoceptor antagonists is further proof of the extremely complex interactions between neurotransmitters in the mechanism of action of modafinil.
GABA and glutamate
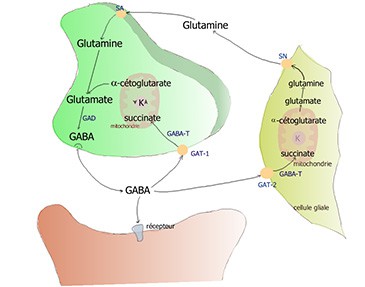
Glutamate is the main excitatory neurotransmitter and GABA is the main inhibitory neurotransmitter in the brain.[131]Petroff, OA. (2002). GABA and glutamate in the human brain. Neuroscientist. 8, 562-573. Enzymes exist in the brain that can catalyse the production of GABA from glutamate and the breakdown of glutamate into GABA, so there is a constant interplay in the balance of these neurotransmitters.
In one study, modafinil reduced GABA release and increased glutamate levels in some areas of the basal ganglia of the brain, which are involved with motor control and routine behaviours, as well as aspects of cognition and learning.[132]Ferraroa et al. (1998). The effects of modafinil on striatal, pallidal and nigral GABA and glutamate release in the conscious rat: evidence for a preferential inhibition of striato-pallidal GABA transmission. Neuroscience Letters Volume 253, Issue 2. Pages 135-138. This may tie in with its efficacy in the treatment of cerebral palsy. Proving that the levels of GABA and glutamate are closely interlinked, the administration of a GABA antagonist counteracted the modafinil-induced increase in glutamate.
Modafinil has also been shown to stimulate the release of glutamate in the hippocampus and some areas of the thalamus in rats.[133]Ferraro et al. (1997). The antinarcoleptic drug modafinil increases glutamate release in thalamic areas and hippocampus. Neuroreport. 8, 2883-2887. Glutamate has a crucial role in cognition, so increased glutamate in these regions of the brain is likely to contribute to modafinil’s nootropic effects.
Advantages over traditional stimulants
Modafinil’s effects are similar in some ways to those of conventional stimulants, but its unique mechanisms of action set it apart.
More targeted effects
Unlike amphetamine, methylphenidate, and other central nervous stimulants that induce wakefulness by general widespread neuronal activation, modafinil is quite selective in its mechanisms and locations of action within the brain. This gives rise to a number of specific benefits of modafinil over its counterparts.
In rats, modafinil and amphetamine both increase the brain’s expression of the gene c-fos, a marker of neuronal activation.[134]Dragunow, M., Faull, R. (1989). The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods. 29, 261-265. But amphetamines activate the cortex and striatum diffusely,[135]Lin, JS., Hou, Y., Jouvet, M. (1996). Potential brain neuronal targets for amphetamine-, methylphenidate-, and modafinil-induced wakefulness, evidenced by c-fos immunocytochemistry in the cat. Proc Natl Acad Sci U S A. 93, 14128–14133. whereas modafinil produces a more localised pattern of c-fos activation focused primarily in various areas of the hypothalamus, which is intricately involved with wakefulness, and the amygdala, which is involved in the processing of emotions.[136]Scammell et al. (2000). Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J Neurosci. 20, 8620-8628.
Amphetamines and similar drugs work by stimulating the sympathetic nervous system (the one responsible for the ‘fight’ part of the ‘fight or flight’ response) and inhibiting the parasympathetic nervous system (the ‘flight’ part) to increase the amount and activity of norepinephrine, serotonin, and dopamine throughout the brain.[137]Lin et al. (1992). Role of catecholamines in the modafinil and amphetamine induced wakefulness, a comparative pharmacological study in the cat. Brain Res. 591, 319-326. This stimulation creates the classic dilated pupils and results in additional strain on the cardiovascular system as well as other systemic side effects, such as nausea. Modafinil does not result in any such systemic sympathetic effects.[138]Duteil et al. (1990). Central alpha 1-adrenergic stimulation in relation to the behaviour stimulating effect of modafinil; studies with experimental animals. Eur J Pharmacol. 180, 49-58.
No disruption of sleep

While amphetamines and other traditional stimulants indisputably improve wakefulness, when the effects wear off the user must make up for lost sleep. Surprisingly, following the use of modafinil, many seem to be able to return to their normal sleep-wake cycle without the need to make up for this lost sleep time.[139]Lagarde et al. (1995). Interest of modafinil, a new psychostimulant, during a sixty-hour sleep deprivation experiment. Clin Pharmacol. Modafinil also has minimal effect on the time taken to get to sleep or on sleep quality,[140]Pigeau et al. (1995). Modafinil, d‐amphetamine and placebo during 64 hours of sustained mental work. I. Effects on mood, fatigue, cognitive performance and body temperature. Journal of Sleep Research Volume 4, Issue 4. Pages 212-228.,[141]Lin et al. (2000). Effects of amphetamine and modafinil on the sleep/wake cycle during experimental hypersomnia induced by sleep deprivation in the cat. J Sleep Res. 9, 89-96. whereas many stimulants are associated with difficulty achieving and maintaining sleep.[142]Gossop, MR., Bradley, BP., Brewis, RK. (1982). Amphetamine withdrawal and sleep disturbance. Drug Alcohol Depend. 10, 177-183.,[143]Bastuji, H., Jouvet, M. (1988). Successful treatment of idiopathic hypersomnia and narcolepsy with modafinil. Prog Neuropsychopharmacol Biol Psychiatry. 12, 695-700.
No high, no crash, no addiction

Also unlike amphetamines, modafinil is not associated with euphoria or any kind of ‘high’; nor does it result in a ‘crash’ as the effects wear off. Cocaine users have reported a complete lack of euphoria with the drug and were easily able to tell the difference between cocaine and modafinil in a test.[144]Rush, CR., Kelly, TH., Hays, LR., Wooten, AF. (2002). Discriminative-stimulus effects of modafinil in cocaine-trained humans. Drug and Alcohol Dependence Volume 67, Issue 3. Pages 311–322. The risk of tolerance or dependence is considered to be very low.[145]Myrick, H., Malcolm, R., Taylor, B., LaRowe, S. (2004). Modafinil: preclinical, clinical, and post-marketing surveillance–a review of abuse liability issues. Ann Clin Psychiatry. 16, 101-109.,[146]Jasinski, DR. (2000). An evaluation of the abuse potential of modafinil using methylphenidate as a reference. J Psychopharmacol. 14, 53-60.
However, it is worth noting that one study cautions of the possible risk of dependence on the basis that modafinil increases dopamine release in an area of the brain known as the nucleus accumbens that is known to be associated with substance dependence.[147]Volkow et al. (2009). Effects of Modafinil on Dopamine and Dopamine Transporters in the Male Human Brain: Clinical Implications. JAMA. 301, 1148–1154.
Rare and mild side effects
Across a wide range of studies evaluating the use of modafinil in varied contexts, the incidence of side effects has been low. For evidence, look no further than the huge range of studies cited in the above sections. In some cases where side effects have been observed, a dose reduction has been effective in alleviating these effects. In the next section, we will consider side effects in more detail.
Side effects and safety concerns
General side effects
In the systematic review of the use of modafinil in healthy individuals that we discussed in some detail earlier, the authors concluded that there were no ‘preponderances for side effects or mood changes’.[148]Battleday, RM., Brem, A.-K. (2015). Modafinil for cognitive neuroenhancement in healthy non-sleep-deprived subjects: A systematic review. European Neuropsychopharmacology Volume 25, Issue 11. Pages 1865–1881. Of the nine studies investigating side effects, seven reported none, with the remainder finding a very low incidence of ‘insomnia, headache, stomach ache or nausea, and dry mouth’. However, these side effects were also observed in the placebo group, suggesting that they may be unrelated to the consumption of the drug.

According to the US FDA, other side effects may include back pain, diarrhoea, nervousness, anxiety, dizziness, rhinitis, and upset stomach.[149]MEDICATION GUIDE PROVIGIL® In the myriad studies looking at the use of modafinil in the treatment of various conditions, the incidence of such side effects has been very low. However, a 2013 UK study evaluating the safety of modafinil across a range of off-label uses stated that ‘individual cases of cardiac, psychiatric and skin events indicated causal associations with modafinil’.[150]Davies, M., Wilton, L., Shakir, S. (2013). Safety Profile of Modafinil Across a Range of Prescribing Indications, Including Off-Label Use, in a Primary Care Setting in England. Drug Safety Volume 36, Issue 4. pp 237–246.
As it stands, the only definitive contraindication to modafinil use listed by the US FDA is known allergy or hypersensitivity.[151]MEDICATION GUIDE PROVIGIL® However, its use in pregnancy should also be avoided. While no controlled studies exist evaluating its effect on foetal development, restricted foetal growth and spontaneous abortion have been reported with modafinil treatment.[152]PROVIGIL® (modafinil) tablets, for oral use, C-IV Initial U.S. Approval: 1998
Lack of evaluation of long-term use
It’s important to note that all studies to date examining the use of modafinil in healthy individuals have looked at the effects of a single dose of modafinil. While the lack of side effects is encouraging, it can in no way be extrapolated to long-term use. In addition, using modafinil in combination with other medications or at higher doses than recommended may create a very different picture.
Risk of substitution for sleep

While no research has ever uncovered exactly why humans (or any animals for that matter) sleep and why it is necessary, the consensus remains that it is critical for learning, memory, neuroplasticity, and general health.[153]Gorgoni et al. (2013). Is Sleep Essential for Neural Plasticity in Humans, and How Does It Affect Motor and Cognitive Recovery? Neural Plasticity Volume 2013, Article ID 103949, 13 pages. It’s hard to imagine it not being a critical function when no animal has ever been shown to be able to survive without it.[154]Cirelli, C., Tononi, G. (2008). Is Sleep Essential? PLOS Biology The point is, no matter how well you feel like you’re functioning on modafinil, you should never use it as a substitute for sleep. That’s one of modafinil’s greatest advantages – that it does allow you to fall into good-quality sleep when the time comes.
Interactions with other medications

Over 90% of the metabolism of modafinil occurs in the liver. Modafinil has been found to induce some enzyme systems in the liver while suppressing others, which has the potential to alter the metabolism of other medications.[155]Robertson, P., DeCory, HH., Madan, A., Parkinson, A. (2000). In vitro inhibition and induction of human hepatic cytochrome P450 enzymes by modafinil. Drug Metab Dispos. 28, 664-671. Notably, it may reduce the effectiveness of contraceptives, though this has not been proven. If you have a medical condition or are taking any medications, check with your doctor before taking modafinil.
In one case report, the administration of modafinil to a man receiving clozapine resulted in toxicity from the latter.[156]Dequardo, JR. (2002). Modafinil-Associated Clozapine Toxicity. The American Journal of Psychiatry Volume 159, Issue 7. Pages 1243-a-1244. The authors attributed this to a metabolic interaction between the drugs.
Fixed drug eruptions
Fixed drug eruptions (FDE) is an umbrella term that describes drug-induced immune-mediated skin diseases characterised by a distinctive syndrome of skin and mucous membrane redness, ulceration and eruptions that vary in severity but can be life-threatening. Reactions such as these tend to occur within the first 5 weeks of treatment with a drug.[157]FDA. Modafinil (marketed as Provigil): serious skin reactions. Postmarketing Reviews. Recognised forms of FDE include Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug rash with eosinophilia and systemic symptoms (DRESS), all of which have been linked to modafinil.
SJS has received the most media attention. While the link to modafinil is indisputable, it is important to know that SJS is extremely rare: in the UK, its incidence has been estimated at around 6.5 cases per million people per year.[158]Jossi, J. (2016). The Epidemiology of Stevens – Johnson Syndrome and Toxic Epidermal Necrolysis in the United Kingdom: A Comprehensive Observational Study. Not only that, but SJS has been observed as a reaction to a wide range of medications, among the most common of which are antibiotics, pain relief, and anti-inflammatory medications.
In Australia, between 2003 and 2008, over 4,000 prescriptions for modafinil were filled, and only 2 serious skin reactions were reported, neither of which was life-threatening.[159]AUSTRALIAN ADVERSE DRUG REACTIONS BULLETIN Volume 27, Number 6, December 2008 By comparison, the US FDA received six cases of severe skin reactions associated with modafinil between December 1998 and January 2007.[160]FDA. Modafinil (marketed as Provigil): serious skin reactions. Postmarketing Reviews.
The reality is that any medication has the potential to cause severe adverse reactions, and modafinil is no different.
Psychiatric reactions

Psychiatric reactions have been reported to occur occasionally with modafinil treatment. These reactions primarily occur in people with a pre-existing history of psychiatric illness, but a few isolated cases have occurred in those without such a history.[161]2008, on the 25 March. “Modafinil: Serious Skin Reactions and Psychiatric Symptoms.” Metronidazole | MIMS Online Modafinil should be used with caution in those with a history of psychosis, depression, mania, or any other psychiatric diagnosis.
Addiction and dependence potential

While modafinil does not produce the high nor the crash that is associated with traditional stimulants and has shown little if any potential for abuse or addiction, this is no guarantee that there may not be some level of dependence in the long term. A few case reports exist describing dependence, though these cases all involved people taking doses above the recommended range, with one patient taking up to 2,500 mg per day.[162]Kate, N., Grover, S., Ghormode, D. (2012). Dependence on supratherapeutic doses of modafinil: a case report. Prim Care Companion CNS Disord. 14.,[163]Krishnan, R., Chary, KV. (2015). A rare case modafinil dependence. J Pharmacol Pharmacother. 6, 49–50.,[164]Cengiz, Mete M., Şenormancı, Ö., Saraçlı, Ö., Atasoy, N., Atik, L. (2015). Compulsive modafinil use in a patient with a history of alcohol use disorder. Gen Hosp Psychiatry. 37, e7-8.
Another concern is that modafinil could end up downregulating our natural cognitive abilities. Among smokers, nicotine deprivation can reduce cognitive function.[165]Harrison, EL., Coppola, S., McKee, SA. (2009). Nicotine deprivation and trait impulsivity affect smokers’ performance on cognitive tasks of inhibition and attention. Exp Clin Psychopharmacol. 17, 91-98. Over time, could we see something similar happen to people who are accustomed to regularly consuming modafinil? Maybe, but it seems unlikely. One study followed narcoleptic patients receiving modafinil for 3 years and found no signs of tolerance.[166]Bastuji, H., Jouvet, M. (1988). Successful treatment of idiopathic hypersomnia and narcolepsy with modafinil. Prog Neuropsychopharmacol Biol Psychiatry. 12, 695-700.
Overdose

Two large retrospective reviews have failed to document any major effects of modafinil overdoses. A 2008 multi-poison centre review uncovered 137 cases,[167]Spiller et al. (2009). Toxicity from modafinil ingestion. Clinical Toxicology. 47:2, 153-156. while a 2010 review using the California Poison Control System database uncovered 87 cases.[168]Carstairs, SD., Urquhart, A., Hoffman, J., Clark, RF., Cantrell, FL. (2010). A Retrospective Review of Supratherapeutic Modafinil Exposures. Journal of Medical Toxicology Volume 6, Issue 3. pp 307–310. In the former, only 17% of patients required inpatient treatment, compared with 44% in the latter. The most common side effects were increased heart rate and blood pressure, insomnia, anxiety, agitation, dizziness, and headache. In a case report of a teenager who ingested 5,000 mg of modafinil, the patient experienced headache, nausea, abdominal pain, insomnia, and transient increased rate and altered electrical activity of the heart.[169]Neuman, G., Shehadeh, N., Pillar, G. (2009). Unsuccessful Suicide Attempt of a 15 Year Old Adolescent with Ingestion of 5000 mg Modafinil. J Clin Sleep Med. 5, 372–373. No changes were noted in the organ or immune function, nor blood pressure.
Dosage, absorption and elimination
The recommended dose of modafinil for on-label uses is usually 100–200 mg/day, taken in the morning. For off-label use, there is of course no official recommended dose, though studies in healthy people have tended to use similar doses to those used on-label. Many online articles and forums suggest that 50 mg is plenty to experience cognitive-enhancing effects.[170]“Modafinil Dosage Guide: 50mg, 100mg, 200mg, or 400mg Dose.” Health, Nutrition, and Nootropics News, 20 Apr. 2018,[171]“Modafinil: Here’s Why Everyone Is Using Smart Drugs.” Bulletproof, 11 Aug. 2017
Many of the studies referenced in this article report that the eugeroic effects of modafinil appear to be dose-independent, while side effects are dose-dependent.[172]Zifko, UA., Rupp, M., Schwarz, S., Zipko, HT., Maida, EM. (2002). Modafinil in treatment of fatigue in multiple sclerosis. Results of an open-label study. J Neurol. 249, 983-987. Some studies even suggested that increased doses may be less effective than lower doses, though this may be tied in with the increased experience of side effects.[173]Rammohan, KW., Rosenberg, JH., Lynn, DJ., Blumenfeld, AM., Pollak, CP., Nagaraja, HN. (2002). Efficacy and safety of modafinil (Provigil) for the treatment of fatigue in multiple sclerosis: a two centre phase 2 study. J Neurol Neurosurg Psychiatry. 72, 179-183.
A small study with eight participants noted increased blood pressure and heart rate in participants receiving an 800 mg daily dose for 7 days, while those receiving 400 mg or 600 mg did not display these side effects.[174]Wong et al. (2013). A Double‐Blind, Placebo‐Controlled, Ascending‐Dose Evaluation of the Pharmacokinetics and Tolerability of Modafinil Tablets in Healthy Male Volunteers. The Journal of Clinical Pharmacology Volume 39, Issue 1. Pages 30-40. However, the study was too small to conclude that either 400 or 600 mg daily doses are safe.
One study of 50 participants receiving a placebo, caffeine, or one of three modafinil doses (100 mg, 200 mg, 400 mg) found no significant difference in performance or alertness between the three different doses of modafinil (or caffeine, interestingly). There was, however, a small non-significant trend towards improved performance at the higher modafinil doses.
A 2012 report on the pharmacokinetics of modafinil showed that modafinil is absorbed well and reaches peak blood concentrations 2 – 4 hours post-ingestion.[175]Robertson, Philmore, Jr., Hellriegel, ET. (2003). Clinical Pharmacokinetic Profile of Modafinil. Clinical Pharmacokinetics Volume 42, Issue 2. pp 123–137. Its half-life is around 12–15 hours. Taking modafinil with food is likely to slow the rate of absorption, but not the overall amount absorbed.[176]Cox, JM., Pappagallo, M. (2001). Modafinil: A gift to portmanteau. American Journal of Hospice and Palliative Medicine® Vol 18, Issue 6.
Modafinil is 90% metabolised and excreted via the liver, with the remainder being eliminated via the kidneys. Elimination can therefore be slower in patients with liver or kidney compromise, and in these patients, modafinil should be used with caution.
Considerations before taking modafinil

There’s no doubt that modafinil is a versatile and effective drug with many potential applications in the treatment of sleep disorders and beyond. Its effects on wakefulness, attention, executive function and physical performance are well documented, which is resulting in ever-growing popularity among students, professionals, and anyone seeking a boost to their everyday cognition – without the side effects or potential for dependence that have plagued users of amphetamines and the like.
But does that mean you should rush out and buy some? Not necessarily. The first complication, of course, is that modafinil legally requires a prescription in Australia, the US, the UK and most other countries. Many users are buying it online without a prescription, and it is rare for customs to intercept it. In general, the worst you can expect to happen is for your package to be seized. The risk of true legal complications is low – but not non-existent. Buy at your own risk.
Could you ask a doctor to prescribe it for you? Of course. But the chance a doctor is going to prescribe a scheduled medication to a person in perfect health is low. In 2016 a new policy was introduced at the Annual Meeting of the American Medical Association (AMA) to discourage the use of prescription drugs in healthy people.[177]“AMA Confronts the Rise of Nootropics.” HIPAA Compliance | American Medical Association, 14 June 2016 This outcome was largely related to the rise of nootropics. But outside of the US, doctors are likely to take the same approach. ‘First, do no harm’ is a central tenet of medicine – don’t prescribe a drug to someone who doesn’t need it!
You could argue that modafinil doesn’t do any harm, but the fact is, we don’t know that for certain. Although the evidence suggests that modafinil is safe and well-tolerated long-term, much remains to be discovered. Even its mechanism is still not fully understood and, as pointed out earlier, studies in healthy people have tended to look at a single dose – not dosing over weeks, months or years.
And although side effects are rare, there’s no debating that they exist. One person’s response to modafinil may be radically different to another’s. Modafinil is certainly not right for everyone. As with all nootropics, if you are thinking about using it, you should consider consulting with a medical professional first.
Finally, there is the ethical aspect of the rise of modafinil and other nootropics. Just as the use of beta blockers to improve performance among classical musicians became widespread in the 1980s as competition ramped up,[178]Lehrer, PM. (1987). A Review of the Approaches to the Management of Tension and Stage Fright in Music Performance. Journal of Research in Music Education Vol 35, Issue 3. we may start to see non-users feeling the pressure to keep up with their modafinil-taking counterparts in the workplace and beyond. This could pose a serious problem not only for those who don’t wish to take cognitive-enhancing substances but for those who can’t afford to. And with the positive effects on attention in pilots and military personnel, it seems possible that the non-use of modafinil may become frowned upon, or even considered dangerous.
With all the information you need to make a considered, informed decision, the rest is up to you.
Buy Modafinil Online Review Comparison Table
| Product | Company | Quantity | Price | Country | Website |
 Modalert, Modvigil | modafinilXL | 10 - 500 pills (100 & 200mg) | $29 - $599 |  AU, USA, Worldwide | Visit Website >> |
Australian or NZ? Click here for a discussion on buying Modafinil online.
Originally posted on June 9, 2018, last updated on October 13, 2023.
References

Leave a Reply